
What Is Corrosion?

Corrosion is a native method that transforms pure metals into a robust synthetic form like oxide or hydroxide. It is the constant of metal because of artificial action with their enclosing.
It degrades and erodes the metals by listless their power, looks, and long-lasting factors. Materials will generally part into two sections which are chemical and physical factors.
- 23 Different Types of Doors
- 13 Types of Houses With Pictures And Advantages and Disadvantages
- What Is Glass Fiber Reinforced Gypsum | Applications of GFRG | Disadvantages of The GFRG Panel
- What Is Sash Window | Types of Sash Windows | Benefits of Sash Window | Historic Window Sash Replacement
- What Is MDF | Advantages of MDF | Disadvantages of MDF | MDF Properties | Application of MDF | How to Waterproof MDF | What Is MDF Made of
Types of Corrosion
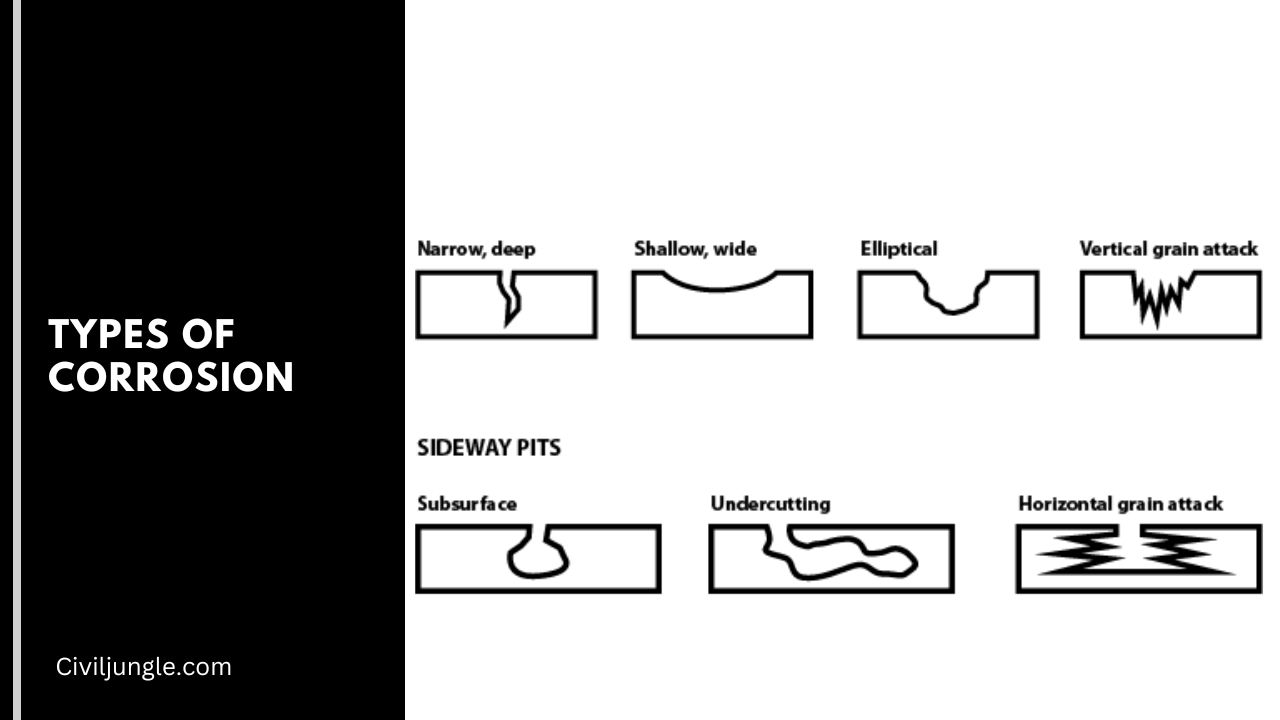
1. Uniform Corrosion

A uniform coat of corruption will produce on the floor in uniform corrosion. The material and unroll over the whole bottom of the materials and this kind of corrosion is abroad spot on materials which are not safe by floor layer such as AL, Zn, and margin will include little materials generally damage by uniform corrosion.
2. Crevice Corrosion

Crevice corrosion happens because little space and interval favor producing liquid and not having a suitable body process. When crevice corrosion occurs, the chance for crevice corrosion adds with many connected fronts. Crevice corrosion will damage by mechanical injury because of the connective sign among the metallic floor and corrosive fluid.
3. Fretting Corrosion

Fretting corrosion happens at the two exposure zone of metals which will connect jointly, and this usually looks when the exposure space will pacify to fall and difference. This corrosion can be straightforward in fastening and engaging, secure floor, etc.
4. Corrosion Fatigue

Fatigue matter will explain as the defeat of matter being replay apply of pressure, and the fatigue of ore will grow in the corrosive climate, then it will call as corrosion fatigue. It can develop by stopping the fatigue refusal of the metal.
5. Intergranular Corrosion

Intergranular corrosion is the corrosion that looks on the granular field, and the seed will not damage in this situation. It seems when there is a notable variation in a reaction opposite pollutant among sources and seed margins.
6. Galvanic Corrosion

Galvanic corrosion happens when two varieties of materials situate and connect in corrosive potassium, and the galvanic pair produced among the two matters are anode and cathode. Galvanic corrosion occurs the materials must be in electrical touch and bare to an electrolyte.
7. Environmental Cracking

Environmental cracking is a corrosion method resulting from a mixture of ecological states damaging the material, and synthetic, pressure, and the strain-connected outcome can combine.
In the below kinds of environment corrosion: corrosion fatigue, hydrogen-induced cracking, stress corrosion cracking, and liquid metal and embrittlement.
8. Erosion Corrosion

Erosion corrosion is an automatic convince crash of a matter floor, and in a corrosive environment, it will damage the joint motion of liquid matters and durable floors. Be on fire at the gash line finishes of the plumber line.
Also, Read: Top 10 Best Cement Companies In India 2022
Example of Corrosion

Several corrosion examples removed from the daily living hood are;
Rust on metals bare to water like the layered metal of the mechanical washer-dryer where the saline environment momentum the erosion backlash and instantly there are crevice and the classic brown mark of oxidation.
It happens while iron rust in the existence of water and oxygen and oxidation is a different material with the chemical formula Fe2O3.XH2O, which will moisten the form of metal.
Corrosion of water pipeline mostly metallic which favor shatter add time, if not to pollute the water with a little dose of rust, afford it a brown color.
The paint of the statue of liberty, because its natural color should not be fresh but copper, the object from which it will create.
But around in the water, the velocity of wind rust it and coats it with new copper rust, the corrosion solution. Below is the chemical formula for copper corrosion
Cu + H2O + CO2 + O2 – Cu (OH) 2 + CuCO3
Corrosion of canning can like that have been in the store everlasting, and brown marks appear in several zones, an unmistakable impact which air corrosion has to start to damage him.
Tarnishing of silver- chemical reaction among the silver and Sulphur carry elements in the air because the silver to tarnish and silver supplied will build while silver material becoming dark because of a coating of silver sulfide. The below chemical formula of metal is
2Ag + H2S – Ag2S + H2
Also, Read: Top 15+ Best Plywood Brands in India
Main Causes of Corrosion

Pollutant in metals- commonly, impurity in metals momentum corrosion because their pollutants function as an invisible electrochemical layer which causes pollution.
In the situation of metals in electrochemical cell- in corrosion, the electrochemical series will be essential and active metals lose electrons sooner and corrode earlier.
The concentration of oxygen- corrosion will mention by developing in oxygen, and an anode will place with a small oxygen concentration, whereas a cathode is a zone with more oxygen concentration. Breakdown occurs as a solution.
Presence of electrolytes- they conduct ions, dilute the salt in waterworks an electrolyte and corrosion will movement while electrolytes will make existence in water.
Moisture in climate- while the water is moist, the existence of humidity with high-pressure movement corrosion because ions get power and start shifting earlier in more pressure, causing them to collide regularly.
Types of Corrosion
- Atmospheric Corrosion
- Erosion Corrosion
- Selective Corrosion
- Uniform Corrosion
- Pitting Corrosion
- Fretting Corrosion
- Stress Corrosion
- Inter-Granular Corrosion
- Corrosion Fatigue
What Is Dry and Wet Corrosion?
Dry corrosion occurs when there is no water or moisture to aid the corrosion, and the metal oxidises with the atmosphere alone. Wet corrosion of metals occurs through electron transfer, involving two processes, oxidation and reduction. In oxidation, the metal atoms lose electrons.
What Is Corrosion Give Three Examples?
The formation of rust on iron, tarnish on silver, and the blue-green patina that develops on copper are all examples of corrosion. Corrosion is defined as the degradation of metals due to an electrochemical process.
What Is Dry Corrosion Example?
Dry corrosion occurs when a metal reacts directly with oxygen. Eg: Burning of magnesium ribbon to produce magnesium oxide. Wet corrosion occurs when a metal reacts directly with oxygen in the presence of water. Eg: Corrosion of iron in water to form rust.
What Is Another Name for Dry Corrosion?
Dry corrosion, sometimes also called chemical corrosion, is a type of corrosion that occurs without moisture or water, which are the main drivers of the conventional types of corrosion. In dry corrosion, metal oxidizes with only the atmosphere, in a process sensitive to temperature.
What Are Advantages of Corrosion?
Corrosion of metals is an advantage as it prevents the metal underneath from further damage. For example: On exposure to air the surface of metal like aluminium and Zinc forms layers of their oxides which are very sticky and impervious in nature and hence act as protective layer.
What Are the Types of Wet Corrosion?
- Crevice Corrosion.
- Erosion Corrosion.
- Galvanic Corrosion.
- General Corrosion.
- Intergranular Corrosion (Igc)
- Pitting Corrosion.
- Stress Corrosion Cracking (Scc)
- Uniform Corrosion.
3 Main Causes of Corrosion
Metal corrodes when it reacts with another substance such as oxygen, hydrogen, an electrical current or even dirt and bacteria.
What Is Corrosion and Its Causes?
Corrosion is a phenomenon of oxidation of metal in the presence of oxygen when it is exposed to external environmental factors which include moisture, acid rain, etc., and chemicals like acids, bases, etc. For example – Rusting of Iron, tarnishing of Silver wares, green coating on Copper vessels.
What Are the Causes and Prevention of Corrosion?
Uncontrolled humidity and corrosive gases are the primary causes of corrosion in industrial environments. Condensation of water vapor on metal surfaces and corrosive gases such as ammonia, chlorine, hydrogen oxides, sulfur oxides, etc., can result in the corrosion of electronic equipment, rusty corners, and corroded parts.
What Are the Causes of Corrosion in Materials?
General corrosion occurs when most or all of the atoms on the same metal surface are oxidized, damaging the entire surface. Most metals are easily oxidized: they tend to lose electrons to oxygen (and other substances) in the air or in water. As oxygen is reduced (gains electrons), it forms an oxide with the metal.
What Causes Corrosion of Steel?
When acidic substances (including water) come in contact with metals, such as iron and/or steel, rust begins to form. Rust is the result of corroding steel after the iron (Fe) particles have been exposed to oxygen and moisture (e.g., humidity, vapor, immersion).
Like this post? Share it with your friends!
Suggested Read –
- 36 Different Types of Plumbing Valve
- Best Concrete Mix for Driveway Repair
- All About Tile Popping | What Is Tile Popping | Reason for Tile Popping | How to Fix Popped-Up Tiles
- All About Transportation of Concrete | What Is Transportation of Concrete | Methods for Transportation of Concrete
- All About Asphalt Flooring | What is Asphalt Flooring | Asphalt Flooring Used | Asphalt Flooring Advantages and Disadvantages
- All About Roof Overhangs | 10 Various Types of Roof overhangs | Standard Roof Overhangs | Overhang Roof Design | Roof overhangs on houses

